
NIH Data Management and Sharing Policy (DMSP)
New NIH Funding Requirement Starting January 25
Beginning in January 2023, Final NIH Policy for Data Management and Sharing (NOT-OD-21-013) will require researchers to include a data management and sharing (DMS) plan in funding applications. In preparation for the policy implementation, NIH has launched a Scientific Data Sharing Website.
The Final DMS Policy applies to all research, funded in whole or in part by NIH, that results in the generation of scientific data. This includes competing grant applications and proposals for contracts that are submitted to NIH on or after the January 25, 2023, submission deadline. The DMS Policy does not apply to research and other activities that do not generate scientific data, including training, infrastructure development, and non-research activities.
Once a grant has been awarded, the DMS Plan becomes part of the award's terms and conditions. NIH expects researchers and institutions to implement data management and sharing practices as described in their approved Plan. Compliance with the DMS Plan, including any updates, may be reviewed during regular reporting intervals.
Applicability
The DMS Policy applies to all research that generates scientific data, including Research Projects, some Career Development Awards, SBIR/STTR, and Research Centers. The DMS Policy does not apply to research and other activities not generating scientific data, including Training Grants, Fellowships, Construction (C06), Conference Grants, Resource (Gs), and Research-Related Infrastructure Programs.
Explore these NIH Introductory Webinars outlining the new requirements.
Source: The University of Alabama at Birmingham
Researchers will be required to submit a DMS plan at the time of application to the funding NIH Institute, Center, or Office (ICO) that outlines how scientific data and related metadata will be managed and shared, including any potential restrictions or limitations.
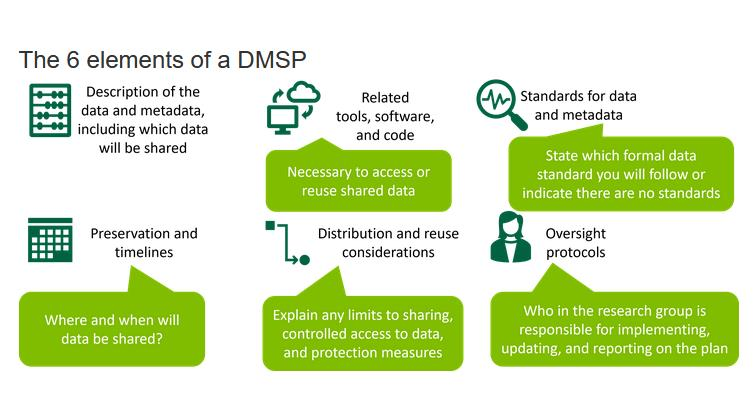
Below are the six required elements and some details on how to address them. NIH has published a Data Management and Sharing Plan Elements Guide which you can read for more information.
Element 1: Data Type
Summarize the types and estimated amount of scientific data expected to be generated in the project. Describe which scientific data from the project will be preserved and shared. NIH does not anticipate that researchers will preserve and share all scientific data generated in a study. Researchers should decide which scientific data to preserve and share based on ethical, legal, and technical factors. The plan should provide the reasoning for these decisions. Briefly list the metadata, other relevant data, and any associated documentation (e.g., study protocols and data collection instruments) that will be made accessible to facilitate interpretation of the scientific data.
Related Resources:
- NIH Sharing Data from Human Participants
- NIH Genomic Data Sharing Policy
- See the Principal Investigator Human Subjects Items to Consider When Developing the DMS Plan (below)
Element 3: Standards
State what common data standards, if any, will be applied to the scientific data and associated metadata to enable interoperability of datasets and resources, and provide the name(s) of the data standards that will be applied and describe how these data standards will be applied to the scientific data generated by the research proposed in this project. If applicable, indicate that no consensus standards exist.
Element 4: Data Preservation, Access, and Associated Timelines
Provide the name of the repository(ies) where scientific data and metadata arising from the project will be archived. Describe how the scientific data will be findable and identifiable, i.e., via a persistent unique identifier or other standard indexing tools. Describe when the scientific data will be made available to other users (i.e., no later than time of an associated publication or end of the performance period, whichever comes first) and for how long data will be available.
Related Resources:
Element 5: Factors affecting access, distribution, or reuse of scientific data, controlled access and privacy considerations
NIH expects that in drafting Plans, researchers maximize the appropriate sharing of scientific data. Describe and justify any applicable factors or data use limitations affecting subsequent access, distribution, or reuse of scientific data related to informed consent, privacy and confidentiality protections, and any other considerations that may limit the extent of data sharing. State whether access to the scientific data will be controlled (i.e., made available by a data repository only after approval). If generating scientific data derived from humans, describe how the privacy, rights, and confidentiality of human research participants will be protected (e.g., through de-identification, Certificates of Confidentiality, and other protective measures).
Related Resources:
- See the Principal Investigator Human Subjects Items to Consider When Developing the DMS Plan (below)
- UCCS DMPS Awareness Handout
- UCCS IRB SOPs (Find the most recent procedures in the SOPs under Manuals and Operating Procedures)
Element 6: Oversight of Data Management and Sharing
Describe how compliance with this Plan will be monitored and managed, frequency of oversight, and by whom at your institution (e.g., titles, roles). This element refers to oversight by the funded institution, rather than by NIH. The DMS Policy does not create any expectations about who will be responsible for Plan oversight at the institution. At UCCS, the PI and their team are responsible for oversight and compliance with their DMSP.
Data Management and Sharing Plan FAQs
If you have specific questions about budgeting for the DMSP, please reach out to osp@uccs.edu
Allowable Costs:
- Curating data
- Developing supporting documentation
- Formatting data according to accepted community standards, or for transmission to and storage at a selected repository for long-term preservation and access
- De-identifying data
- Preparing metadata to foster discoverability, interpretation, and reuse
- Local data management considerations, such as unique and specialized information infrastructure necessary to provide local management and preservation
- Preserving and sharing data through established repositories (ex: data deposit fees). If proposing multiple repositories, costs associated with each may be included
- Note: Personnel (salary and fringe) effort specific to DMS must be included as part of the DMS Costs line item in the detailed R&R budget. (If a modular budget, it must be detailed in the Additional Narrative Justification).
Unallowable Costs:
- Infrastructure costs that are included in institutional overhead (for instance, Facilities and Administrative costs)
- Costs associated with the routine conduct of research, including costs associated with collecting or gaining access to research data.
- Costs that are double charged or inconsistently charged as both direct and indirect costs
If an award is made, the approved DMS Plan will become a term and condition of the award and the PI is responsible for managing and sharing data as described in the DMS Plan. During the funding period, compliance with the Plan will be determined by the NIH Institute or Center.
Effective October 12, 2023, prior approval requests must be submitted through eRA Commons by a UCCS approved signing/authorized official (NOT-OD-23-185). These requests must be submitted at least 30 days in advance of the requested change. Please contact osp@uccs.edu as soon as possible if you believe you need to make changes to your DMS Plan. To learn more, see NIH's announcement for DMS Plan Prior Approval Capability.
Prior approval from the NIH Program Officer along with the revised DMS Plan are required when the following changes occur to the plan:
- New Scientific Direction
- Change in Data Repository
- Timeline Revision
Compliance with the Plan, including any Plan updates, may be reviewed during regular reporting intervals (e.g., at the time of the annual RPPR). Please contact osp@uccs.edu when submitting a NIH RPPR.
Resources
UCCS and CU Resources
- UCCS DMSP Awareness Sheet
- Finding an Open Repository for Data Research from Kramer Family Library
- For assistance contact Norah Mazel
- Sample DMS Plans based on NIH examples
- Other CU Resources:
NIH Resources
- NIH Resources to Prepare Applicants for New Data Management Sharing Policy - Includes sample plans, a two-part webinar series, FAQs, and a list of data repositories
- NIH Sample Plans
- NIH Budgeting for Data Management and Sharing
- NDA Cost Estimation Tool
- NIH Sharing Data from Human Participants
- NIH Supported Data Repositories
- Resources to help you find an appropriate repository for your research
- NIH Data Management and Sharing Tips Resources
- NIH Data Management and Sharing FAQ
- NIH Data Management and Sharing Plan vs. Resource Sharing Plan
- NIH Links to Supplemental Information
- NOT-OD-21-014 – Elements of an NIH Data Management and Sharing Plan
- NOT-OD-21-015 – Allowable Costs for Data Management and Sharing
- NOT-OD-21-016 – Selecting a Repository for Data Resulting from NIH-Supported Research
- NOT-OD-23-070 – NHLBI Supplement to the NIH Policy for Data Management and Sharing (DMS Policy Comparison Table)
Other Resources
- DMPTool - Provides templates that can be used in developing Data Management Plans for grant applications to U.S. federal funding agencies (this tool may produce a document that is beyond the page limit)
- OSF Working Group on DMSP Guidance - Includes policy readiness checklist for librarians and researchers and other resources